Introduction
The advancement of implantable medical devices has significantly transformed modern healthcare by enabling continuous monitoring, precise diagnostics, and targeted therapeutic intervention. From pacemakers and neurostimulators to biosensors and cochlear implants, these devices must operate reliably within the harsh and complex environment of the human body. To ensure long-term functionality and biocompatibility, device manufacturers face the crucial challenge of shielding delicate electronic components from moisture, bodily fluids, and biological reactions.
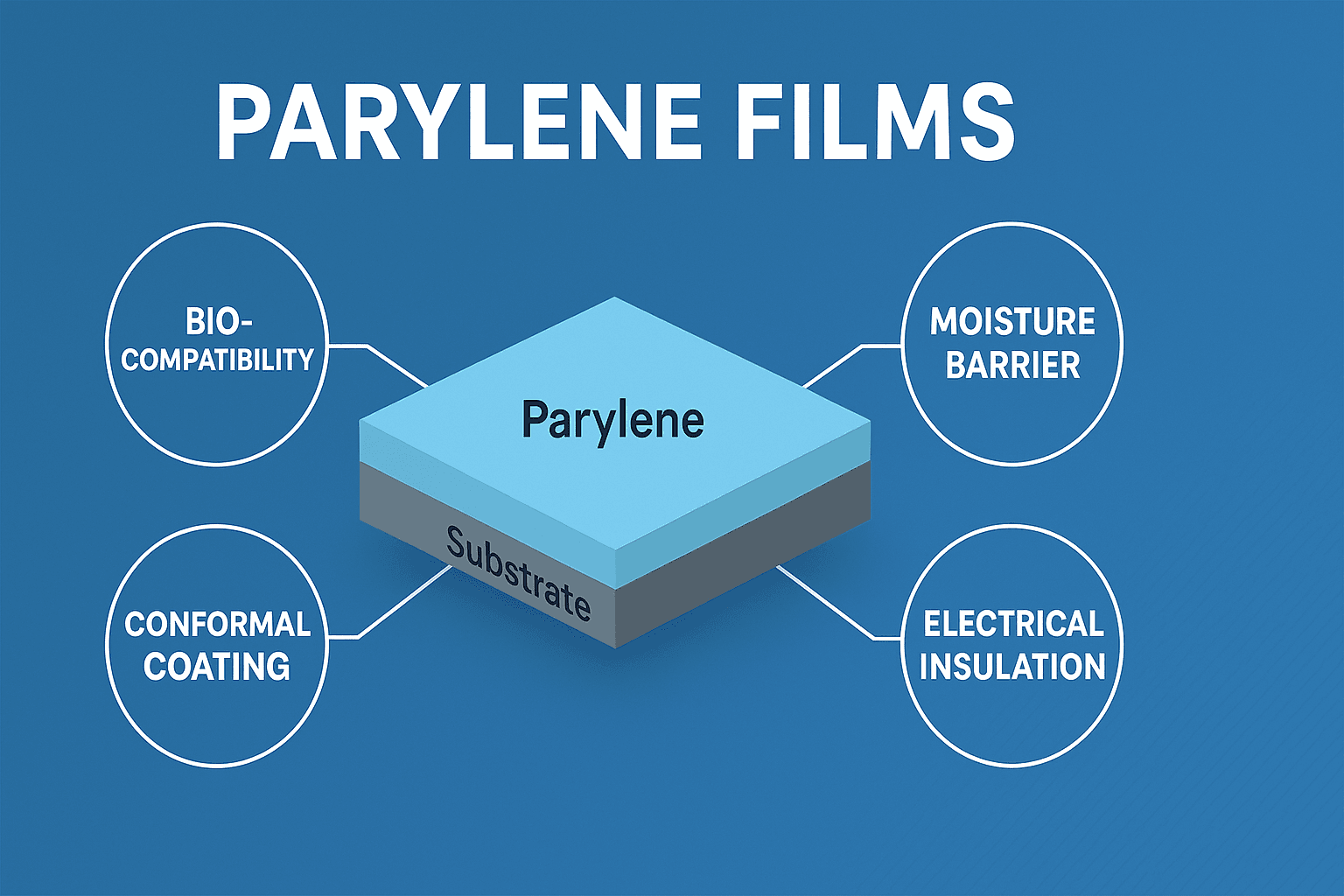
Among various protective coatings, Parylene films have emerged as the gold standard in conformal coatings for implantable medical devices. Known for their exceptional biocompatibility, chemical inertness, and conformal coverage, medical-grade Parylene coatings provide critical protection that enhances both the performance and lifespan of implantable technologies.
This article explores in detail how Parylene films serve as vital protectors of implantable devices, examining their properties, benefits, application techniques, and contributions to advancing medical innovation.
1. What is Parylene?
Parylene is a generic name for a series of poly(p-xylylene) polymers. These polymers are synthesized through a unique chemical vapor deposition (CVD) process that enables them to form ultra-thin, pinhole-free, and highly uniform coatings on virtually any substrate.
Common Types of Medical Parylene:
- Parylene C: The most widely used variant for medical applications, valued for its balance of barrier properties and biocompatibility.
- Parylene N: Offers superior dielectric properties and high purity but less barrier protection compared to Parylene C.
- Parylene HT (AF-4): Fluorinated version with improved thermal stability, often used in high-temperature or radiation-sensitive environments.
Parylene coatings are typically applied in thicknesses ranging from a few hundred nanometers to several microns, depending on the protection level required.
2. Why Implantable Devices Need Protection
Implantable devices are subjected to a hostile internal environment that includes:
- Continuous exposure to bodily fluids such as blood, saline, and interstitial fluid.
- Temperature fluctuations near 37°C, with occasional changes due to fever or inflammation.
- Mechanical stresses from muscle contractions, organ movement, or external pressure.
- Electrochemical corrosion from ionic activity within the body.
- Immune responses, leading to encapsulation or tissue inflammation.
Without adequate protection, sensitive device components such as sensors, electrodes, circuit boards, and batteries may experience degradation, electrical shorts, or failure—compromising patient safety.
3. How Parylene Protects Implantable Devices
3.1 Biocompatibility and Bio-Stability
Parylene C is USP Class VI certified and complies with ISO 10993 biocompatibility standards, meaning it can remain inside the body without causing adverse reactions such as toxicity, irritation, or inflammation. It is bio-inert and resists degradation even after prolonged exposure to bodily fluids.
3.2 Moisture and Chemical Barrier
One of the core functions of Parylene is its ability to prevent moisture ingress. Its low water vapor transmission rate (WVTR) ensures that even sub-micron coatings can shield sensitive electronics from corrosion and short circuits. Additionally, Parylene resists penetration by acids, bases, and other chemicals found in physiological environments.
3.3 Conformal Coverage
Unlike dip or spray coatings, Parylene is applied using a CVD process in a vacuum chamber. The gaseous monomer penetrates even the most complex geometries, forming a truly conformal, pinhole-free layer around the entire surface—including undercuts, crevices, and sharp edges. This 3D coverage ensures consistent protection across the whole device.
3.4 Electrical Insulation
Parylene exhibits excellent dielectric strength, making it ideal for coating electronic circuits within implantable devices. This insulating property ensures electrical isolation, protects against current leakage, and maintains signal integrity in sensors and communication modules.
3.5 Thin, Lightweight, and Flexible
Despite its robustness, Parylene films are incredibly thin and add negligible weight or volume. This is critical for miniaturized implants such as neurostimulators or glucose monitors, where space constraints are paramount. Its flexibility also accommodates dynamic mechanical stresses within the body without cracking or delaminating.
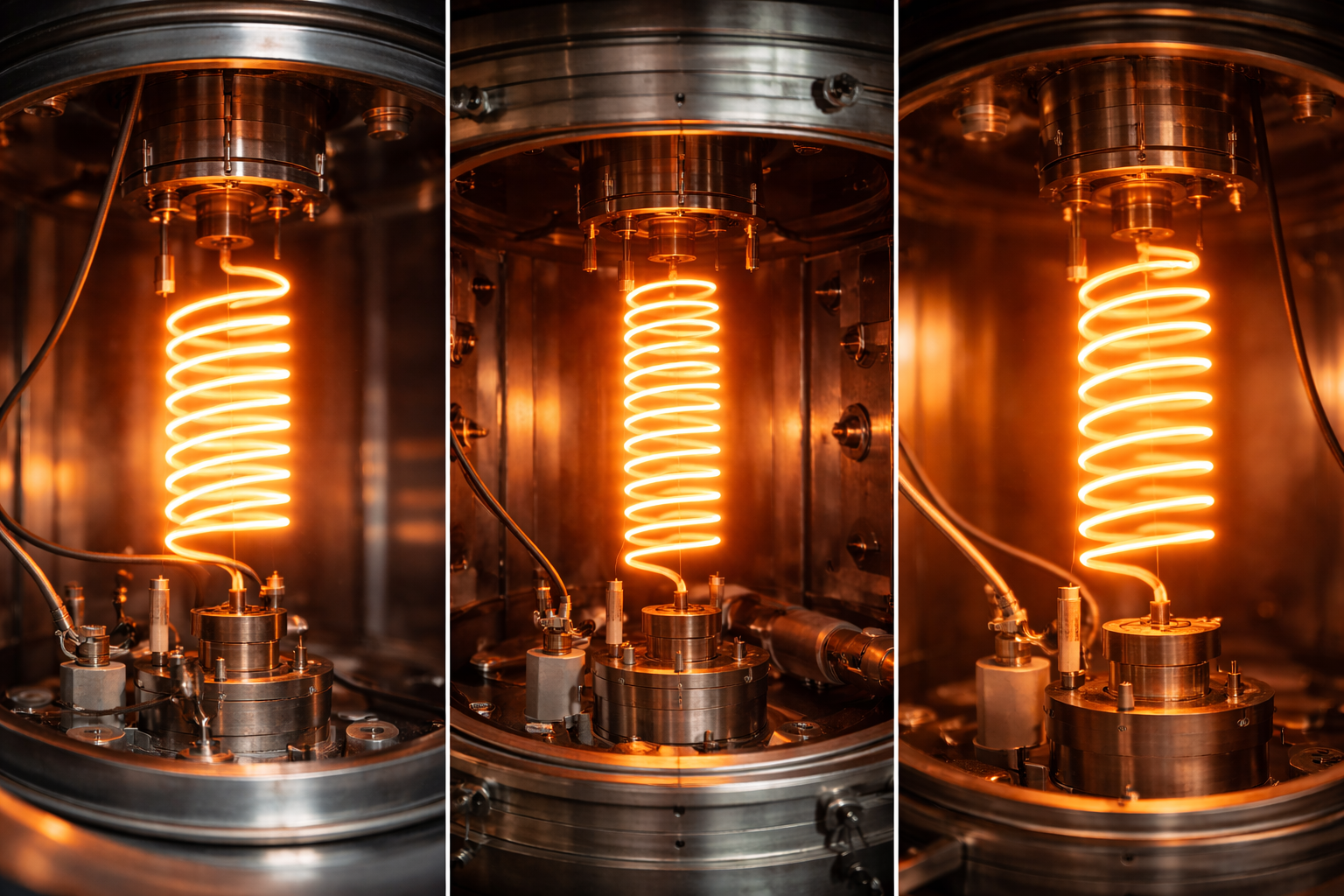
4. Application Process of Parylene Coating
Parylene is deposited through a multi-step vapor deposition process:
- Vaporization: The solid dimer (e.g., Parylene C) is vaporized under vacuum at around 150°C.
- Pyrolysis: The vaporized dimer is cracked into monomeric gas at high temperatures (~650°C).
- Deposition: The monomer gas enters a cooler chamber containing the substrate, where it polymerizes spontaneously on all exposed surfaces.
This process occurs at room temperature, avoiding thermal damage to heat-sensitive components and enabling use on a wide range of materials including plastics, metals, ceramics, and elastomers.
5. Use Cases of Parylene in Implantable Devices
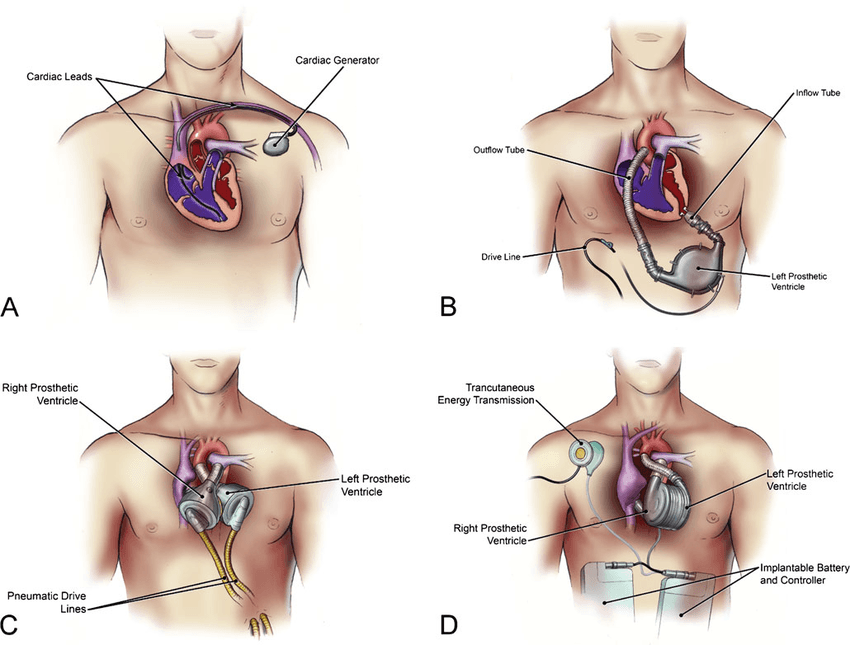
5.1 Cardiac Devices
- Pacemakers and Implantable Cardioverter Defibrillators (ICDs) rely on Parylene to isolate and protect electronic circuitry from moisture-induced failure.
- Parylene enhances longevity and ensures patient safety by preventing corrosion of leads and connectors.
5.2 Neurostimulators
- Devices such as spinal cord stimulators and deep brain stimulators require high-reliability coatings for electrodes and microelectronic systems.
- Parylene’s bio-stability ensures that stimulation parameters remain consistent over years of implantation.
5.3 Cochlear Implants
- These devices interface with the auditory nerve and include sensitive electronics.
- Parylene protects against fluid ingress and electrical leakage while maintaining mechanical flexibility.
5.4 Biosensors and Glucose Monitors
- Implantable sensors for continuous glucose monitoring or pH detection need long-term operation in vivo.
- Parylene prevents biofouling and ensures accurate readings by providing a stable, non-reactive barrier.
5.5 Orthopedic and Drug Delivery Implants
- In devices with embedded electronics (e.g., programmable pumps, stimulators), Parylene ensures function without introducing toxic leachables.
- Drug-eluting implants benefit from Parylene’s controlled permeability properties.
6. Comparative Advantages Over Other Coatings
| Coating Material | Moisture Barrier | Biocompatibility | Conformality | Process Temp. | Common Failures |
|---|---|---|---|---|---|
| Parylene C | Excellent | Excellent | Excellent | Room Temp. | Minimal |
| Epoxy | Moderate | Fair | Poor | High (~150°C) | Cracking, delam. |
| Silicone | Good | Good | Moderate | Moderate | Swelling |
| Polyurethane | Moderate | Fair | Moderate | High | Degradation |
| Teflon (PTFE) | Good | Good | Poor | High | Poor adhesion |
Parylene clearly offers a superior combination of properties for sensitive medical implants.
7. Challenges and Considerations
Despite its benefits, Parylene coating requires careful consideration during device design and manufacturing:
- Masking: Areas such as electrodes that must remain exposed require precise masking, which can be labor-intensive.
- Surface Preparation: Proper cleaning and adhesion promoters (e.g., A-174 silane) are often necessary to ensure long-term bond strength.
- Inspection: Due to its transparency and thinness, visual inspection is challenging; specialized methods like UV inspection or electrical testing are needed.
- Cost: The CVD process and equipment are more costly compared to simpler coating methods, though this is justified by performance in critical applications.
8. Regulatory and Industry Standards
Medical-grade Parylene coatings must comply with stringent regulatory requirements:
- ISO 10993: Biological evaluation of medical devices.
- USP Class VI: Tests for systemic toxicity, intracutaneous toxicity, and implantation safety.
- FDA 510(k) and PMA: Used in devices cleared or approved for human implantation.
- RoHS and REACH: Environmental and chemical safety regulations.
Top Parylene coating service providers often operate ISO 13485-certified facilities to meet medical manufacturing standards.
9. Emerging Trends and Innovations
9.1 Parylene in Microfluidics and MEMS
The rise of implantable microelectromechanical systems (MEMS) and lab-on-a-chip platforms is fueling demand for ultra-thin, biocompatible coatings. Parylene offers ideal surface properties for microscale structures.
9.2 Antimicrobial Parylene
Researchers are developing Parylene films embedded with silver nanoparticles or doped with iodine to offer antimicrobial protection—particularly useful in infection-prone implants.
9.3 Parylene Patterning
Innovative techniques such as laser ablation and plasma etching are enabling selective patterning of Parylene, allowing multi-material integration and functional surface engineering.
10. Conclusion
As implantable devices continue to evolve in sophistication and miniaturization, the need for reliable, biocompatible, and high-performance protective coatings becomes ever more critical. Medical-grade Parylene coatings fulfill this role with unmatched effectiveness—offering superior moisture barrier, electrical insulation, and conformal coverage without compromising biocompatibility.
By enabling long-term functionality and patient safety, Parylene is not just a protective film but a vital enabler of medical innovation. As materials science and device integration continue to advance, the role of Parylene in next-generation implantables will only grow stronger—safeguarding lives at the intersection of medicine and technology.
You May Also Want to Know
- Is Parylene FDA approved for implants?
Yes, Parylene C is widely used in FDA-approved devices and meets biocompatibility requirements. - How long can Parylene last inside the body?
Decades, depending on the application. Its chemical inertness ensures long-term stability. - Can Parylene be applied to flexible devices?
Yes, it maintains adhesion and flexibility even on dynamic, soft substrates. - Is Parylene coating toxic?
No, medical-grade Parylene is bio-inert and does not release harmful substances. - What’s the typical thickness of Parylene on implants?
Usually between 1–10 microns depending on barrier needs. - Can Parylene be used with wireless implants?
Absolutely. It protects antennas and circuits without interfering with signal transmission. - What happens if Parylene delaminates?
Delamination is rare but can expose components to failure. Proper adhesion protocols prevent it. - How is quality ensured for Parylene coatings?
Through thickness measurement, electrical testing, and adhesion evaluation. - Does Parylene allow drug permeability?
It can be tuned for permeability, useful in drug-eluting devices. - Who provides Parylene coating services?
Major providers include Specialty Coating Systems (SCS), Para Tech Coating, and HZO, among others.