Introduction
Europium(III) oxide (Eu₂O₃), a rare earth sesquioxide, plays a pivotal role in modern vacuum deposition techniques, particularly in thermal and e-beam evaporation processes. As industries continue to demand advanced optical, electronic, and photonic devices, the significance of specialized materials like Europium(III) oxide evaporation materials is rapidly growing. From red phosphors in display technology to potential applications in spintronics, Eu₂O₃ has carved a niche in high-precision thin film engineering.
This article explores the material’s physical and chemical properties, manufacturing processes, usage in evaporation techniques, compatibility with substrates, and specific industrial applications. It also offers detailed insights into the handling and storage of Eu₂O₃ evaporation materials and provides practical guidance for maximizing performance in deposition environments.
1. What is Europium(III) Oxide?
1.1 Chemical Structure and Formula
Europium(III) oxide, commonly abbreviated as Eu₂O₃, is a sesquioxide compound composed of europium ions in a +3 oxidation state and oxygen. It is the most thermodynamically stable oxide form of europium and appears as a reddish-white or pink powder under standard conditions.
- Molecular formula: Eu₂O₃
- Molar mass: 351.926 g/mol
- Crystal system: Monoclinic (cubic at high temperature)
- Bandgap: ~4.4 eV (wide bandgap)
Eu₂O₃ belongs to the broader family of lanthanide oxides, exhibiting characteristic luminescence properties that make it valuable in optoelectronics.
1.2 Physical and Chemical Properties
| Property | Value |
|---|---|
| Appearance | Pink to white crystalline powder |
| Melting Point | ~2,290 °C |
| Density | ~7.42 g/cm³ |
| Vapor Pressure | Low, suitable for high-temp evaporation |
| Solubility in Water | Insoluble |
| Magnetic Behavior | Paramagnetic |
These properties make it ideal for vacuum-based evaporation processes, especially under controlled atmospheric conditions such as inert or reducing gases.
2. Manufacturing of Europium(III) Oxide Evaporation Materials
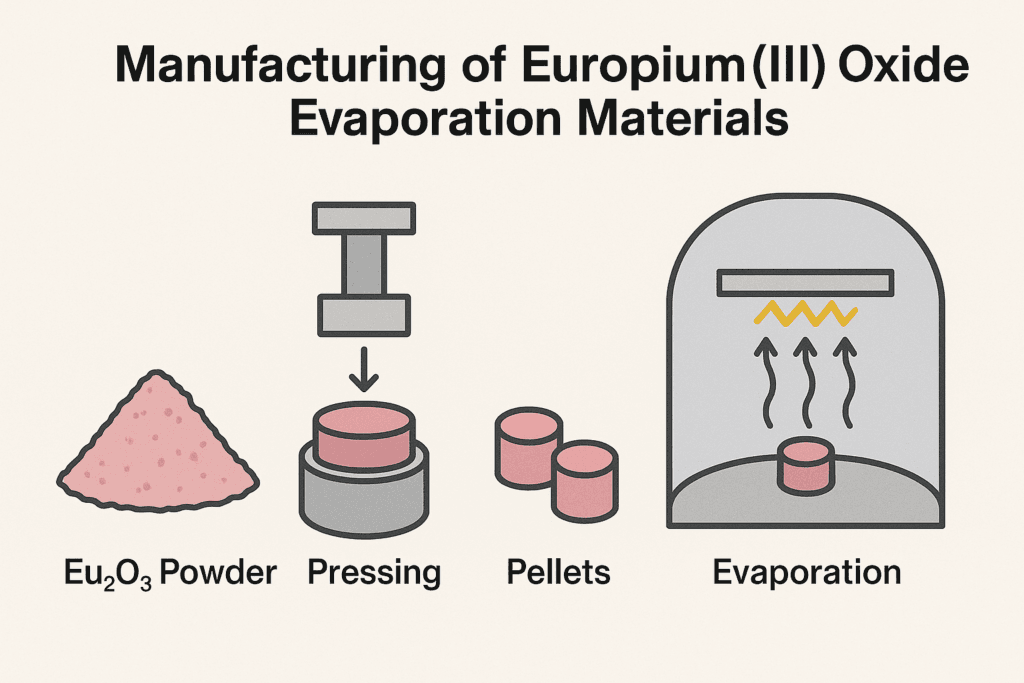
2.1 Raw Material Sources
Europium is extracted primarily from bastnäsite and monazite ores, where it is found in trace amounts alongside other rare earth elements (REEs). After solvent extraction and ion exchange purification, Eu is oxidized to Eu₂O₃ in a controlled environment.
2.2 Purification Process
To produce high-purity evaporation materials for thin film applications, especially in photonics or semiconductor industries, the following steps are essential:
- Calcination: Converts europium nitrate or carbonate to oxide.
- Reduction/oxidation control: Ensures the correct oxidation state (Eu³⁺).
- Vacuum sintering: Enhances density and crystallinity.
- Grain selection and sieving: Prepares uniform powder or granules.
Purity levels of 99.9% (3N), 99.99% (4N), and 99.999% (5N) are common in advanced applications. Higher purity minimizes contamination and optimizes the optical properties of deposited films.
2.3 Form Factors for Evaporation
Eu₂O₃ evaporation materials are supplied in various forms based on the target application and type of evaporation system:
- Granules (1–5 mm)
- Pellets (tablet-pressed or sintered)
- Pieces/Chunks
- Powders (for boat or crucible loading)
- Custom-shaped targets (for e-beam crucibles)
Sintered or pressed Eu₂O₃ pellets are particularly favored in e-beam evaporation systems, while granules and powders are common in thermal evaporation.
3. Thermal and Electron Beam Evaporation Techniques
3.1 E-Beam Evaporation
source. This technique provides:
Compatibility with high-melting-point materials like Eu₂O₃
Precise thickness and stoichiometry control
Vacuum level: 10⁻⁵ to 10⁻⁷ Torr
Deposition rate: 0.1 to 5 Å/s (adjustable)
Substrate temp: Typically 100–500 °C
Care must be taken to prevent “spitting” or explosive boiling due to trapped gas pockets, often mitigated by preconditioning the material and using rotating crucibles.
3.2 Thermal Evaporation
Though less common for Eu₂O₃ due to its high melting point, thermal evaporation using tungsten boats or alumina crucibles is still feasible for low-rate deposition or under optimized low-pressure settings. A high current is passed through a boat loaded with Eu₂O₃, sublimating the oxide in vacuum.
4. Applications of Europium(III) Oxide Thin Films
4.1 Optoelectronics and Phosphor Technology
Eu₂O₃ is widely used as a red phosphor activator due to its strong emission at ~611 nm when excited with UV light. This characteristic emission finds application in:
- Cathode ray tubes (CRT)
- Plasma display panels (PDPs)
- White LEDs (Eu³⁺ doping in Y₂O₃:Eu or YAG)
Thin films prepared via evaporation offer higher purity and more uniform luminescent layers compared to traditional sintering methods.
4.2 Semiconductor and Dielectric Layers
Thanks to its wide bandgap (~4.4 eV) and high breakdown strength, Eu₂O₃ thin films are explored for:
Gate dielectrics in CMOS transistors
Charge storage layers in memory devices
Photodetectors and UV filters
Deposition via e-beam ensures precise thickness control, critical for device reproducibility.
4.3 Spintronics and Magnetic Materials
Though not a traditional magnetic material, Eu₂O₃ exhibits interesting paramagnetic properties and is studied in:
- Spin-polarized electron sources
- Dilute magnetic semiconductors (DMS) when doped with other REEs or transition metals
- Quantum computing concepts involving rare earth magnetic ordering
5. Deposition Parameters and Process Optimization
| Parameter | Optimal Range |
|---|---|
| Base Pressure | < 10⁻⁶ Torr |
| Deposition Rate | 0.1–3 Å/s |
| Source-to-Substrate Distance | 15–30 cm |
| Crucible Material | Alumina or graphite (inert) |
| Substrate Temp | 200–400 °C (depends on film use) |
| Post-annealing | Optional (600–800 °C in O₂ for crystallization) |
Substrate compatibility:
- Glass (soda-lime, quartz)
- Silicon wafers
- Sapphire (Al₂O₃)
- MgO, YSZ, and other oxides
Controlling the oxygen partial pressure during or after deposition enhances film quality and minimizes oxygen vacancies.
6. Packaging, Storage, and Handling
Eu₂O₃ is chemically stable, non-reactive with air or moisture under normal conditions. However, high-purity evaporation materials require careful handling to maintain performance:
- Store in airtight containers with desiccants to prevent moisture adsorption.
- Avoid contamination from tools or hands.
- Use ceramic tweezers and gloves during loading.
- Pre-bake or degas materials before deposition to minimize outgassing.
7. Market Trends and Supply Outlook
7.1 Global Demand Drivers
The demand for Eu₂O₃ evaporation materials is primarily driven by:
- LED and display industries (phosphors)
- Semiconductor innovation (dielectrics, sensors)
- Research and development in quantum and spintronic materials
7.2 Challenges
- Limited europium availability (rare even among rare earths)
- Geopolitical supply risks (China dominates REE supply chains)
- Processing complexity and cost
7.3 Opportunities
- Recycling of phosphor waste for rare earth recovery
- Nanostructured Eu₂O₃ films for advanced sensors
- Increased adoption of ALD and PVD for atomic-layer control of films
8. Alternatives and Doping Approaches
While pure Eu₂O₃ is valued for its strong red emission and optical properties, researchers have developed doped or composite materials to tailor its behavior:
- Eu₂O₃:Y₂O₃ – Enhances emission efficiency in phosphors
- Eu₂O₃:ZnO – Transparent conducting oxide with red emission
- Eu-doped HfO₂/Al₂O₃ – Charge trap memories
Such combinations can also lower the melting point or improve evaporation characteristics, opening doors to hybrid deposition systems.
9. Frequently Asked Questions (FAQs)
Q1: What is the recommended crucible material for Eu₂O₃ evaporation?
Answer: Alumina and graphite crucibles are ideal due to their thermal stability and chemical inertness.
Q2: Can Eu₂O₃ films be deposited on plastic substrates?
Answer: Yes, but only using low-temperature techniques like pulsed laser deposition (PLD) or RF sputtering. Thermal evaporation may damage polymers due to high temperatures.
Q3: How is film thickness monitored during evaporation?
Answer: A quartz crystal microbalance (QCM) is typically used to monitor deposition rate and thickness in real time.
Q4: Does Eu₂O₃ require reactive oxygen during deposition?
Answer: Usually no, as Eu₂O₃ is already in an oxidized state. However, oxygen backfill (~10⁻⁴ Torr) can improve film stoichiometry and optical properties.
Q5: What is the typical film color of evaporated Eu₂O₃?
Answer: It ranges from pale pink to translucent, depending on film thickness and substrate.
Conclusion
Europium(III) oxide evaporation materials are vital for cutting-edge thin film deposition in photonics, display technology, and beyond. Their unique luminescent, dielectric, and paramagnetic properties make them highly desirable across industries requiring high precision, purity, and performance. With increasing demands for miniaturization, efficiency, and advanced functionality, Eu₂O₃-based thin films are poised to contribute significantly to next-generation electronic and optoelectronic devices.
As the materials science community continues to innovate deposition techniques and material combinations, Europium(III) oxide remains a cornerstone of rare earth oxide applications in vapor-phase technologies. For manufacturers, researchers, and engineers, understanding its properties and behavior during evaporation is essential for unlocking its full potential.