Introduction
Li₀.₃₈La₀.₅₆TiO₃ (LLTO) is a perovskite-structured oxide well known for its exceptional lithium-ion conductivity and stability. In recent years, it has become a critical material in the development of all-solid-state lithium batteries, thin-film electrolytes, and next-generation micro-power devices. When fabricated as a sputtering target with a copper backing plate, LLTO ensures both excellent film uniformity and superior thermal management during deposition.
What Is Li₀.₃₈La₀.₅₆TiO₃?
Li₀.₃₈La₀.₅₆TiO₃ belongs to the family of lithium lanthanum titanates, which adopt a perovskite-type ABO₃ crystal structure. The partial substitution of La³⁺ for Li⁺ in the lattice generates lithium vacancies, enhancing ionic mobility. As a result, LLTO exhibits ionic conductivities on the order of 10⁻³–10⁻⁴ S/cm at room temperature—comparable to some liquid electrolytes.
Key properties:
- Crystal structure: Perovskite (orthorhombic)
- Ionic conductivity: ~10⁻³ S/cm (at 25 °C)
- Chemical stability: High, with excellent resistance to oxidation and moisture
- Applications: Solid-state lithium batteries, thin-film electrolytes, electrochemical sensors, and dielectric films
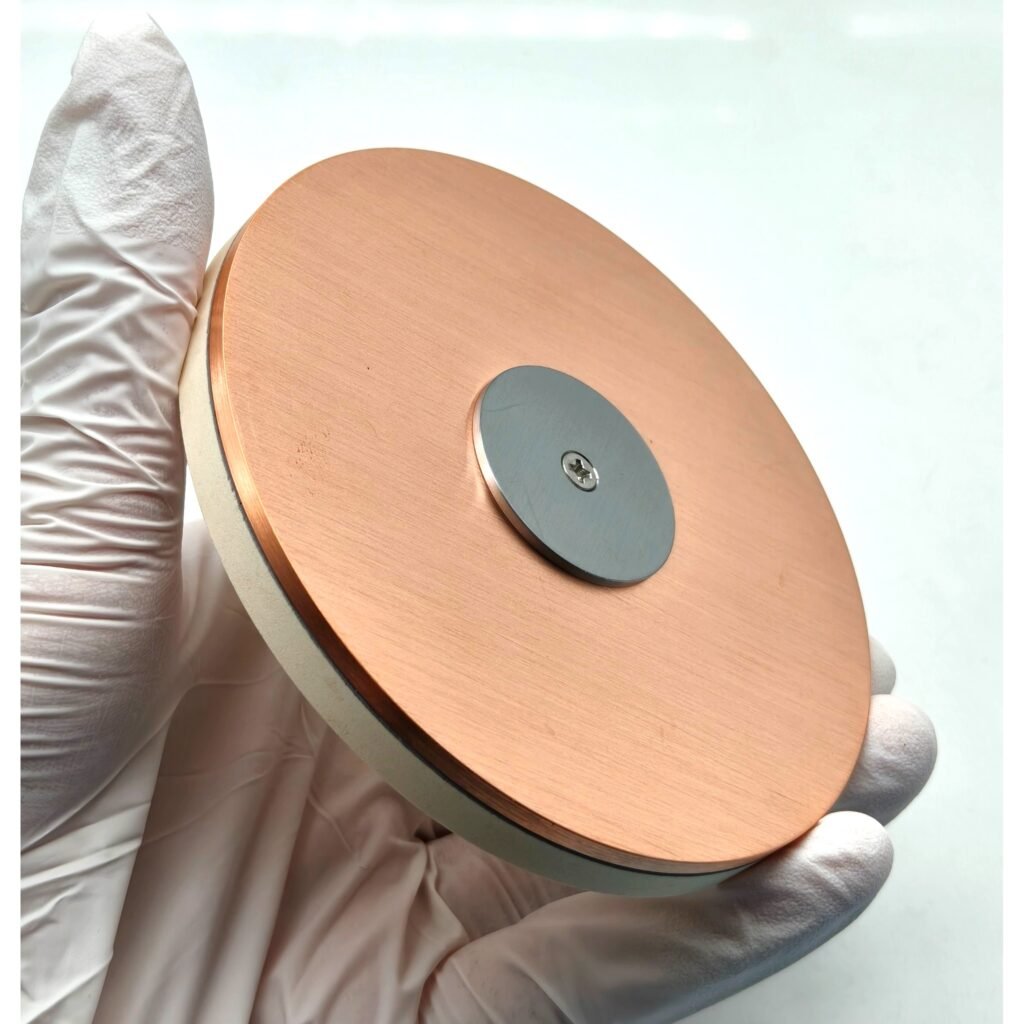
Advantages of a Copper Backing Plate
During RF or DC magnetron sputtering, ceramic oxide targets such as LLTO tend to exhibit low thermal conductivity. By bonding the target to a 2 mm copper backing plate, heat generated during plasma bombardment is efficiently dissipated, preventing cracking and delamination.
Benefits include:
- Enhanced heat transfer during sputtering
- Reduced target warping and cracking
- Improved bonding strength and mechanical stability
- Extended target lifetime and uniform erosion zone
Copper’s high thermal conductivity (~400 W/m·K) makes it the preferred choice for most oxide or compound sputtering targets that operate under medium to high power conditions.
Film Deposition and Applications
Li₀.₃₈La₀.₅₆TiO₃ thin films are typically deposited using RF magnetron sputtering under an oxygen-argon atmosphere. Optimized deposition parameters yield dense, uniform films with smooth surface morphology and high ionic conductivity after annealing (typically 600–800 °C).
Primary application areas:
| Application | Function of LLTO Thin Film |
|---|---|
| All-Solid-State Li Batteries (ASSLBs) | Solid electrolyte layer between cathode and anode |
| Micro-power Devices | Electrolyte for thin-film microbatteries |
| Electrochromic Windows | Ion-conductive layer enabling reversible color change |
| Sensors & Capacitors | Dielectric layer with high stability and low leakage |
In lithium battery research, LLTO films are often combined with cathode materials like LiCoO₂ or LiFePO₄, creating fully solid microbatteries with improved safety and long cycle life.
Manufacturing and Bonding Process

The Li₀.₃₈La₀.₅₆TiO₃ Sputtering Target is typically produced by hot pressing or sintering high-purity oxide powders (Li₂CO₃, La₂O₃, TiO₂) under controlled atmospheres to ensure stoichiometric balance and dense microstructure.
After machining to precise dimensions (e.g., Ø2″ × 3 mm), the ceramic target is bonded to a copper backing plate using indium bonding or elastomer bonding techniques, depending on the sputtering power and cooling configuration.
Typical specifications:
| Parameter | Value |
|---|---|
| Composition | Li₀.₃₈La₀.₅₆TiO₃ |
| Purity | 99.9% (3N) |
| Form | Ceramic disc bonded to Cu backing plate |
| Diameter | 1″–2″ (customizable) |
| Thickness | 3–5 mm |
| Bonding method | Indium bonded to 2 mm Cu plate |
Quality and Customization
At Thin Film Materials (TFM), each LLTO sputtering target undergoes strict density and impurity testing to ensure high ionic performance and stable sputtering rates. Custom compositions (e.g., Li₀.₅₀La₀.₅₀TiO₃ or Li₀.₃₀La₀.₆₀TiO₃) and bonding configurations (e.g., molybdenum or titanium backing plates) are also available upon request.
Quality control includes:
- ICP-MS impurity analysis (< 50 ppm total metallic impurities)
- SEM microstructure examination for uniform grain size
- XRD verification of perovskite phase purity
- Thermal cycling tests for bonding integrity
Why Choose Li₀.₃₈La₀.₅₆TiO₃ for Thin-Film Electrolytes?
- High Ionic Conductivity: Enables fast Li-ion transport across interfaces.
- Excellent Thermal Stability: Maintains crystalline phase during deposition.
- Chemical Compatibility: Stable with common electrode materials.
- Reduced Leakage Current: Suitable for long-term operation in micro-devices.
- Eco-friendly & Non-flammable: Safer alternative to liquid electrolytes.
Related Products
- Li₇La₃Zr₂O₁₂ (LLZO) Sputtering Target – garnet-type electrolyte for solid-state batteries
- Li₃PO₄ Sputtering Target – thin-film electrolyte and buffer layer material
- LiNbO₃ Sputtering Target – piezoelectric and optical applications
- LiCoO₂ Sputtering Target – cathode thin-film deposition
You May Also Want to Know
Q1: What is the difference between LLTO and LLZO?
LLTO has a perovskite structure, while LLZO is a garnet-type oxide. LLZO offers higher bulk conductivity but LLTO provides smoother, denser films at lower sputtering temperatures.
Q2: Can LLTO films be deposited on flexible substrates?
Yes. When sputtered at low temperatures (< 400 °C), LLTO films can adhere well to glass, silicon, and polymer substrates.
Q3: How is indium bonding different from elastomer bonding?
Indium bonding provides superior thermal conduction for high-power sputtering; elastomer bonding allows easier re-bonding and is more tolerant of thermal stress.
Q4: What causes target cracking during sputtering?
Thermal mismatch between the ceramic target and backing plate. Proper bonding and controlled power ramp-up prevent this issue.
Q5: What is the recommended base pressure for sputtering LLTO?
Typically below 5 × 10⁻⁶ Torr, with Ar/O₂ gas flow ratios between 9:1 and 4:1 depending on film stoichiometry.
Q6: Can I request custom dimensions or compositions?
Yes. Custom diameter, thickness, and La/Li ratio can be manufactured based on research requirements.
For inquiries:
📧 sales@thinfilmmaterials.com
🌐 www.thinfilmmaterials.com


